0
1079
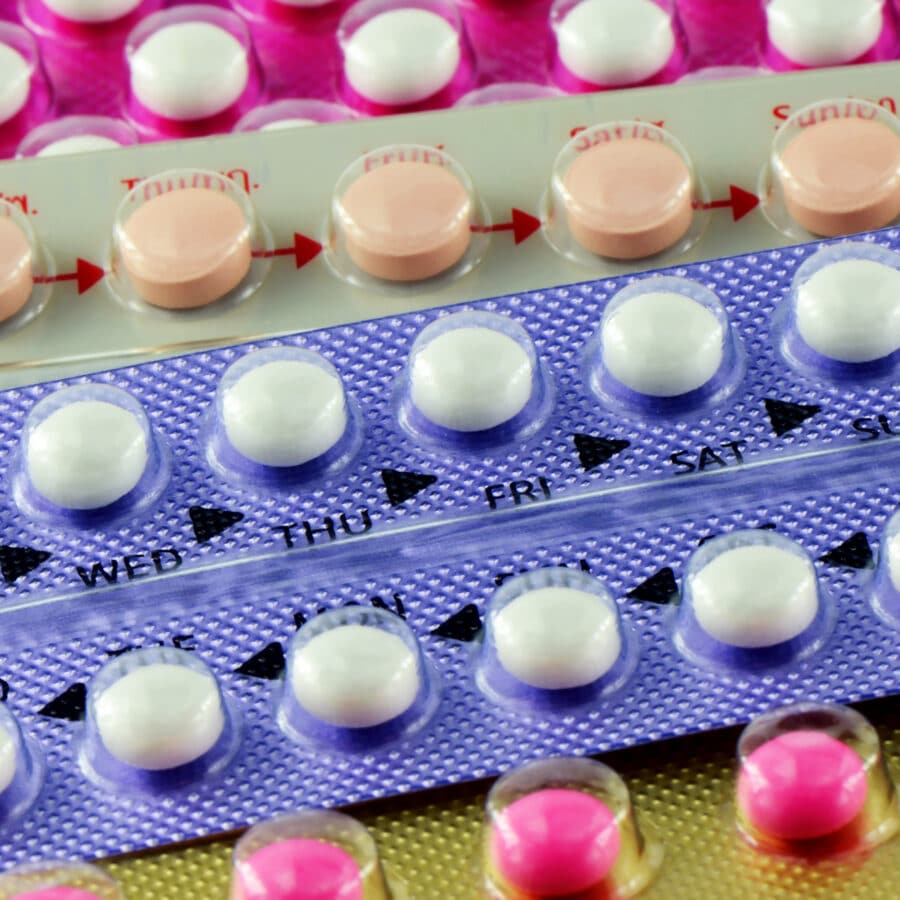
Incorrectly Packaged Oral Contraceptives Recalled Amid Unintended Pregnancy Concerns
The Food and Drug Administration (FDA) recently announced the voluntary recall of two different types of Pfizer oral contraception tablets after the manufacturer found that some packs of the medication may contain the wrong number of inert or active ingredient... Read more