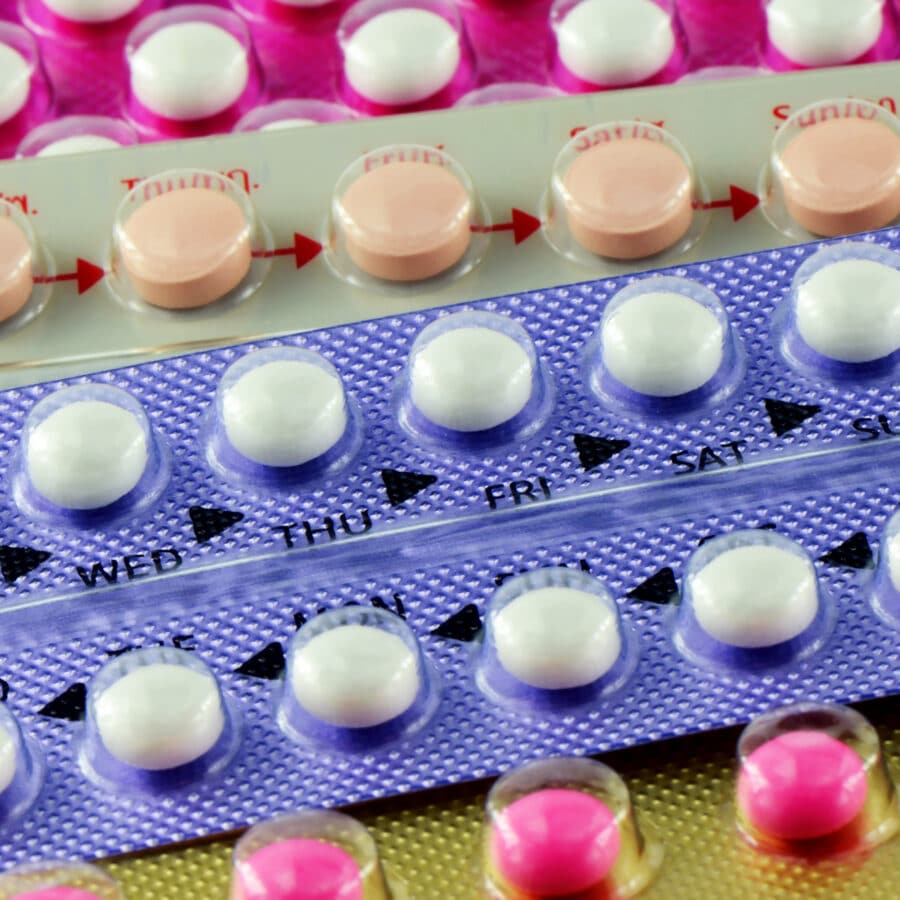
The Food and Drug Administration (FDA) recently announced the voluntary recall of two different types of Pfizer oral contraception tablets after the manufacturer found that some packs of the medication may contain the wrong number of inert or active ingredient tablets and that they may also be packaged out of sequence.
The federal safety resource explains that 14 lots of Lo/Ovral-28 Tablets and 14 lots of the generic Norgestrel and Ethinyl Estradiol tablets were recalled from the U.S. market. A Pfizer press release explains that the 28 lots recalled equal approximately 1 million packs of the medication.
The specific lot numbers affected by the recall can be found with the FDA recall announcement. Although the FDA explains the packaging defects do not put women in any immediate health risks, those who have taken either of these oral contraceptives in the affected lots may have been left unprotected against pregnancy.
Manufactured and packaged by Pfizer Inc., these pills were sold by Akrimax Pharmaceuticals. The recall notice explains these pills are distributed nationally to pharmacies, clinics, and warehouses.
These two brands of oral contraception come in blister packs with 21 active-ingredient tablets and seven inactive-pills. The FDA explains that it is essential patients receive the correct dosage of this product in order to avoid an unplanned pregnancy.
The FDA recommends consumers who used the improperly packaged birth control pills begin using a non-hormonal form of contraception at once. Furthermore, patients with these pills are told to notify their physicians and return them to the pharmacy.